Introduction Large Scale ADC
Antibody-drug Conjugates (ADCs) represent a potent therapeutic for targeted delivery of toxins to specific cell types. ADCs are composed of a monoclonal antibody (mAb) conjugated to a drug molecule via a linker. The use of mAbs results in the delivery of the cytotoxic payload to only the targeted cell type, and because of this ADCs have shown promise as an effective site- specific cancer therapy (1). Two ADCs, Kadcycla® (Genentech/Roche) and Adcetris® (Seattle Genetics), have been approved by the FDA for use in Her2-positive metastatic breast cancer and relapsed Hodgkin’s Lymphoma or systemic anaplastic large cell lymphoma respectively. There are more than 50 ADC candidates currently in clinical development, with more than 10 entering Phase II and III clinical trials (2). However, large-scale production of ADCs has been hampered by a number of factors, including the lack of efficient and consistent conjugation of antibody to the desired toxic compound; inconsistent antibody internalization; and unstable linkers that release the toxin prior to arrival at the target tissue. MabPlex offers state of the art solutions to these problems to allow robustly, large-scale production of ADCs with predictable and consistent conjugation efficiency using a variety of linkers.
The Benefits of the MabPlex CMO/CRO Facility
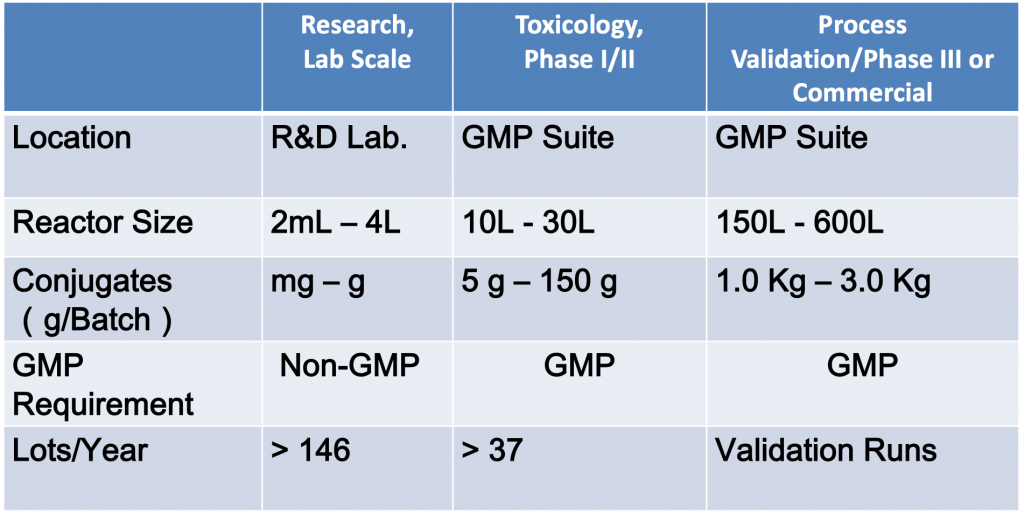
The biggest problems in ADC manufacturing are scalability and consistency. MabPlex utilizes state of the art facilities that allow GMP-compliant production of large batches of ADC, with a reactor size of up to 600L. MabPlex has also optimized eleven parameters that result in the minimal waste of mAbs, toxin, and reagents, potentially saving millions of dollars during the validation / optimization process. Table 1 illustrates the production capabilities at MabPlex.
MabPlex is currently developing and testing a number of ADCs targeting five different tumor-specific antigens. CO103 is the first ADC approved by the CFDA for Phase 1 clinical trials in China. Figure 1 shows the progress of various ADC projects at MabPlex.

Multiple Validated Antibody-Linker-Payload Combinations Available
Designing a functional mAb – linker – payload conjugate is a complex process. MabPlex can assist in determining the best mAb and linker combination for a given functionality. Tethering the drug payload to the mAb can be done using a variety of different linkages. Cysteine-, Lysine-, Thiol-based or site-specific linkages are appropriate for different antibody-drug complexes. Thiol-bridge and site-specific linkages can be engineered to be cleavable or non-cleavable, allowing untethering of the drug payload inside the cell. MabPlex offers all four types of linkages, allowing superior customization of ADC conjugation site and delivery system.
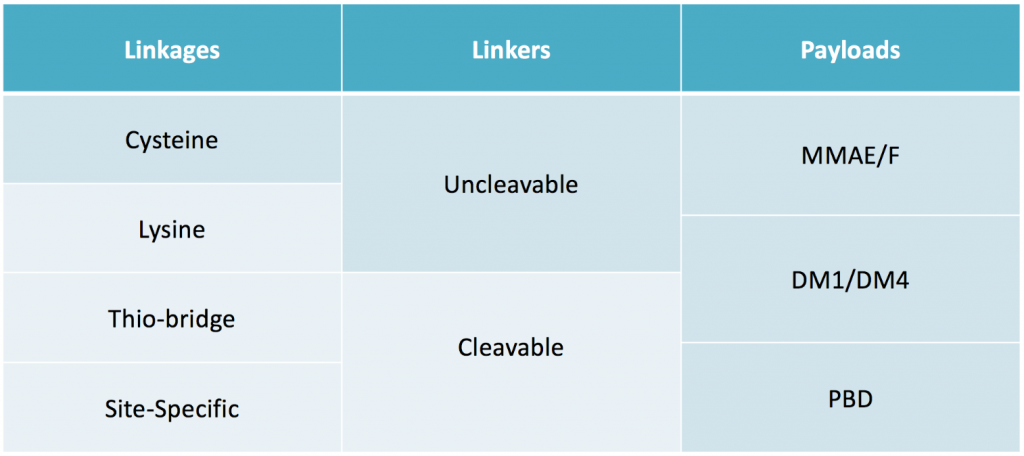
Multiple Validated Antibody-Linker-Payload Combinations Available
Conjugation efficiency of the payload drug is vitally important in generating functional ADCs. Two to four drug molecules are generally required to achieve optimal toxicity. Conjugation of too many drug molecules may result in the ADC itself triggering an immune response, resulting in its elimination from the body prior to reaching the target site. The conjugation process at MabPlex consistently results in an optimal drug-antibody ratio (DAR) of just under four.
MabPlex uses state of the art equipment to determine the DAR distribution for each batch of ADCs. Both native mass spectrometry (native- MS) and Hydrophobic Interaction Chromatography – High Performance Liquid Chromatography (HIC-HPLC) can be used to evaluate DAR with high accuracy (3).
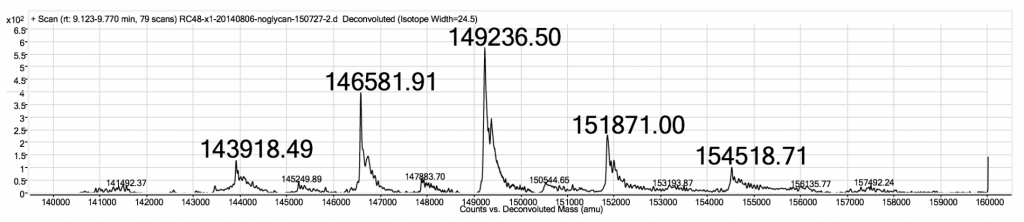
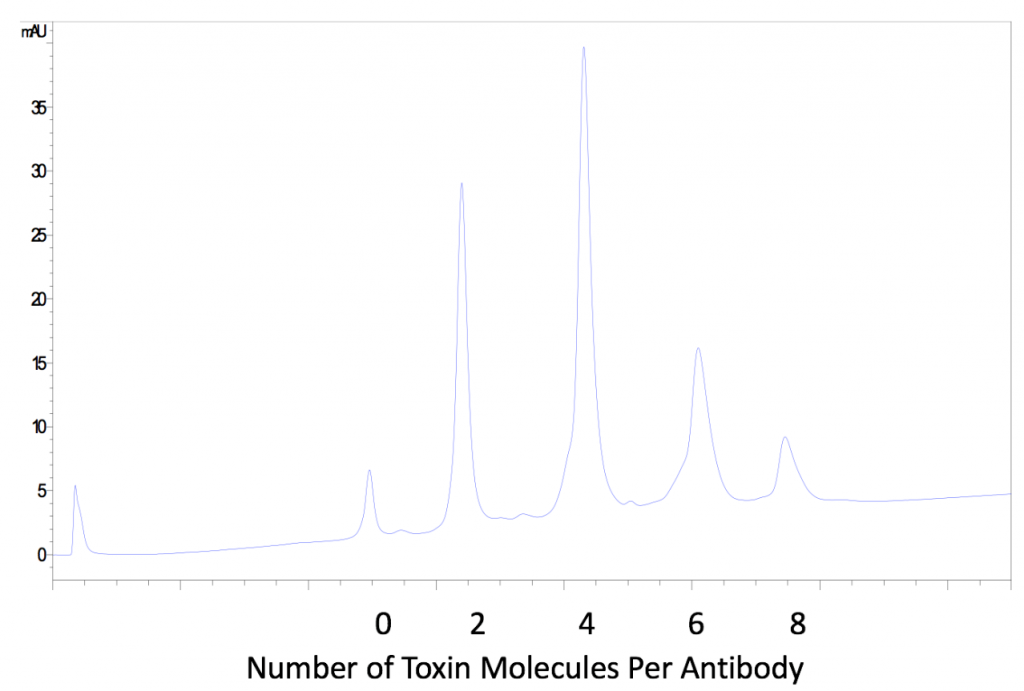
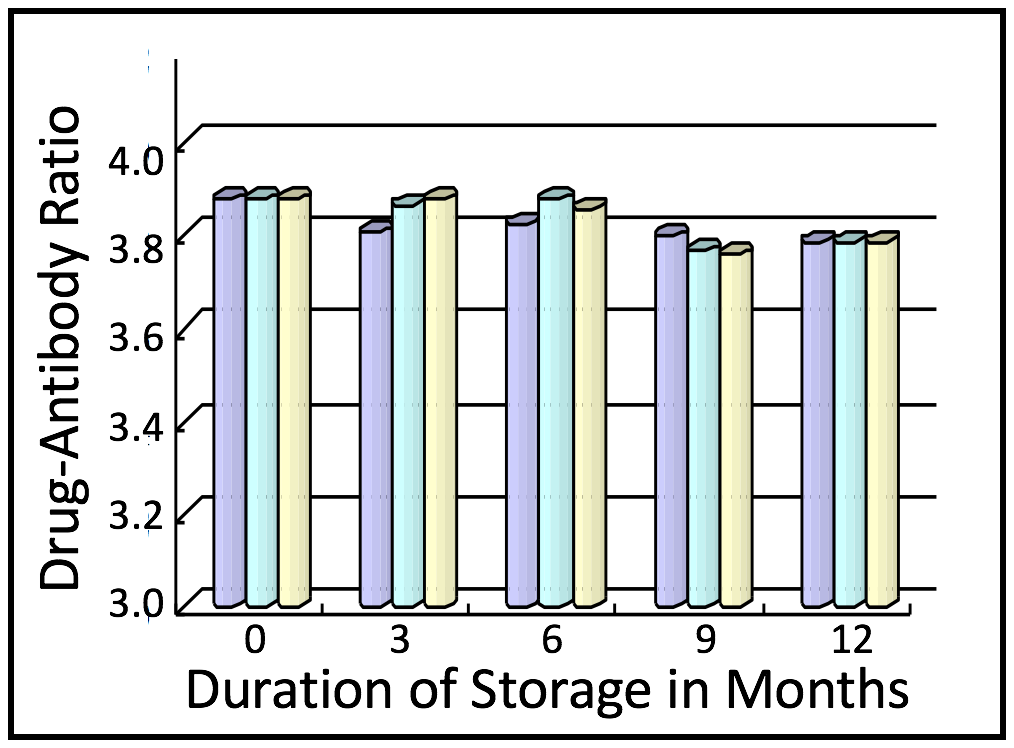
MabPlex ADCs Exhibit High Batch-to-Batch Consistency
ADCs must be able to survive long term storage as well as the process of lyophilization and rehydration with no loss of payload. MabPlex ADCs show excellent retention of payload after both lyophilization and storage at 4oC.