Careful management, integrity and compliance
Regulatory Affairs
MabPlex assists you to navigate complex regulatory processes and advance your program into the clinic and commercial. The CMC core team has more than 20 years of experience in biological and our global RA expertise offers comprehensive services with deep understanding of global regulations and extensive experience in IND\IMPD and BLA filing for biological drugs and biosimilars.
- Regulatory filing strategy and execution
- Meetings (pre-IND/EOPⅡ/Pre-BLA) with agency and investigational applications (IND/IMPD/CTA), amendments and supplements
- Marketing applications (BLA-PHSA 351(a)/ 351(K)/MAA)
- Support for regulatory dossier submissions around the world in both eCTD and non-eCTD formats:
- eCTDs writing, formatting and submitting
CMC Strategy, Management and Communication
- Drug development CMC strategy consulting
- CMC gap analysis
Medium Manufacturing

CelluPro is MabPlex owned medium manufacturing company specializing in the development, production (OEM production) of high-quality serum free medium for mammalian cells culture with the production capacity of about 40 tons powder medium per year, equivalent to 1.4 million liters of liquid medium.
By virtue of the excellent product quality and performance, the medium products of CelluPro have been widely recognized for cell line development and cell culture process development.
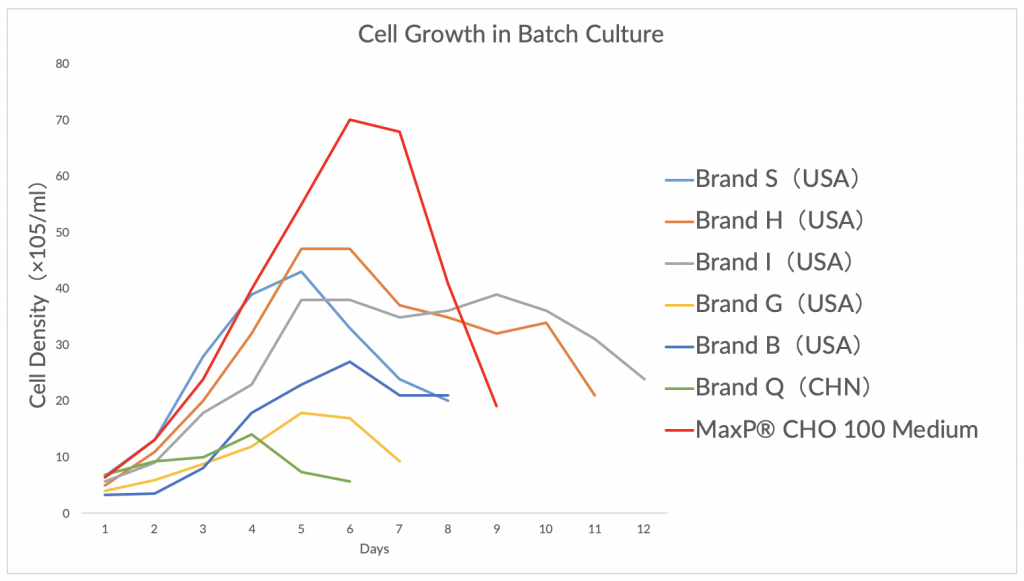
MaxP® CHO 100 Medium
Perfect performance for different cell lines derived from CHO-K1/ DG44/ DXB11/ CHO-S expressing different type of therapeutic protein.
In fed-batch culture, the peak cell density could reach above 2 x 107 cells/ml. The titer of fusion protein or monoclonal antibody could reach to 2~7g/L.
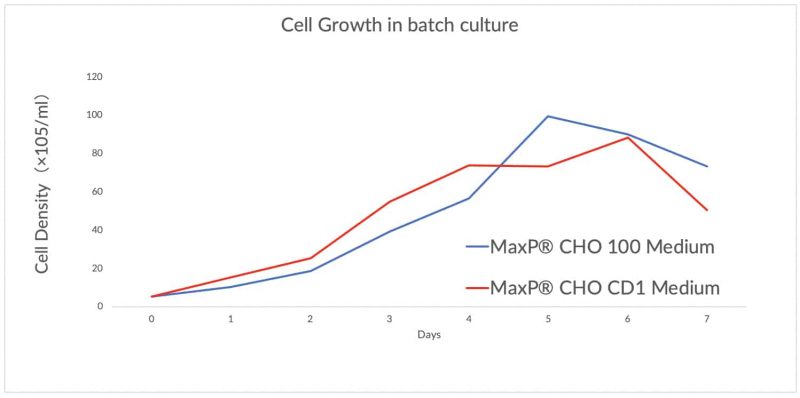
MaxP® CHO CD1 Medium
MaxP® CHO F100 Medium
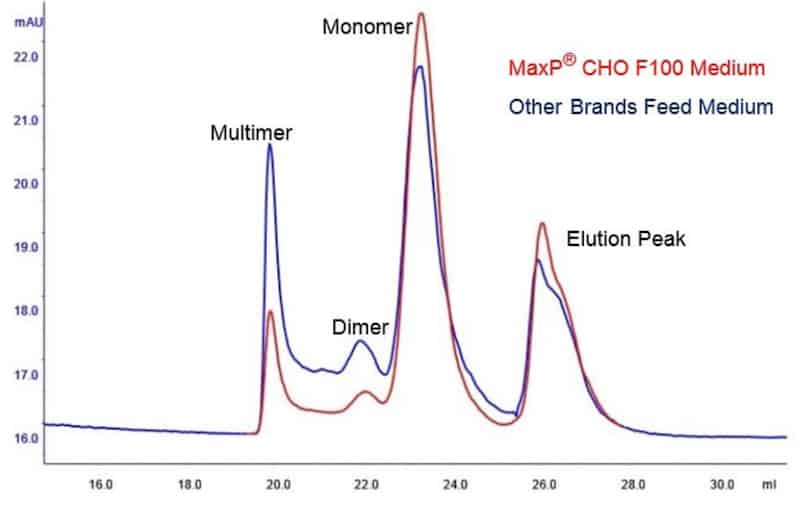
An excellent feed medium for CHO cells to obtain high cell density and titer in feed batch cell culture for antibody and fusion protein expression.
Perfect performance in minimize the aggregates of fusion protein in cell culture process.
SEC Analysis of Fusion Protein in Cell Culture